HC: A Novel Copolymer for Sustained Drug Delivery
- Field
- Drug Delivery
- Patent
- IP00562
Key Problem and Market Opportunity
- Even though chitosan is a versatile compound for sustained drug delivery systems in formats like hydrogel, sponge and powder, being insoluble in water, hydrophilic in nature and the need of an acidic medium to be functional have greatly limited its applications
- Advanced wound dressing and care product is one of the growing markets for sustained drug delivery technologies
- Market size for advanced wound care products is US$ 2.4B in 2014 for US alone, expected to reach US$ 3.3B in 2019
- HC-based wound care products may be classified as Class I medical devices
Key Advantages of the Technology
- Low cytotoxicity
- Long shelf life
- Provide a pH-neutral and stable environment for drug release
| Chitosan | HC | |
|---|---|---|
| Water solubility | ✗ lipophilic drugs | ✓ High |
| Potential to deliver fragile drugs | ✗ Limited due to the need to dissolve chitosan in acidic solutions. The capacity to deliver pH-sensitive drugs is limited. | ✓ High due to the possibility to dissolve HC in neutral solutions |
| Potential to deliver water-soluble drugs | ✗ Low | ✓ High |
| lipophilic drugs | ✗ Limited due to its hydrophilic nature. Lipophilic drugs can hardly mix well with chitosan solutions | ✓ High due to the possibility of HC to homogeneously disperse the lipophilic drug molecules |
ACS Applied Materials & Interfaces, 7(19), 10501–10510.
Keys:
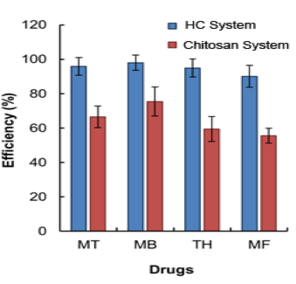
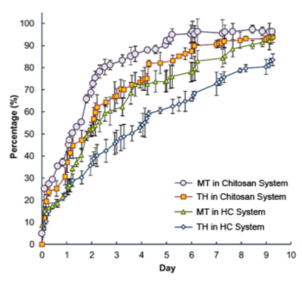
MT: Metronidazole; MB: Methylene Blue; TH: Tetracycline Hydrochloride; MF: Mometasone Furoate
Potential Product and Services
- Wound dressing, care and antiseptic products
- Cosmetic products
- Skin care products
Development Status and IP Strength
- Both gel-type and film-type prototypes are available for drug loading and delivery test
- Prototypes demonstrate effective mixing, encapsulation and loading capability
- US Application No. 15/130,368 and PCT Application No. PCT/CN2016/080313