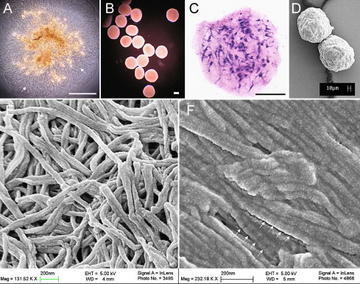
MSC Microspheres for Regenerative Medicine
- Field
- Therapeutic Biologics
- Patent
- IP00213
Key Problem and Market Opportunity
•Stem cell-based therapy (SCT) presents a promising approach for regenerative medicine and tissue engineering
•Using mesenchymal stem cells (MSC) in SCT is more socially and ethically acceptable than embryonic stem cells
•However, nowadays there is no effective delivery device for SCT to have satisfactory functional outcomes
Key Advantages of the Technology
•Self-assembled collagen-MSC microspheres
•Controlled sphere sized: 150-300µm in diameter
•MSC number in the spheres can be controlled for various applications
•Encapsulated MSCs are protected by a dense meshwork of collagen fibrils creating a physiologically relevant permissive environment
Potential Product and Services
Cell-based therapy
Development Status and IP Strength