Neutrophil Elastase (NE) and Proteinase 3 (PR3) as Diagnostic Biomarkers for Autoimmune Diabetes
- Field
- Diagnostics
- Reference No.
- IP00517
Key Problem and Market Opportunity
- Type 1 diabetes (TID) is a progressive autoimmune disease, in which the beta cells that produce insulin are slowly destroyed by the body’s own immune system. TID may have catastrophic onset. It is advantageous for early detection of TID, both for management and treatment.
- As of 2014, 422 million people have diabetes worldwide, of which TID accounts for around 10%.
- In current practice, islet autoantibodies serve as biomarkers for TID. However, in some cases, the autoantibodies cannot distinguish TID with Type II Diabetes. Besides, the diagnostic sensitivity of these markers is around 50%-60%, and the markers are not suitable for early detection.
- Therefore, there are needs of new biomarkers for early detection autoimmune diabetes.
Key Advantages of the Technology
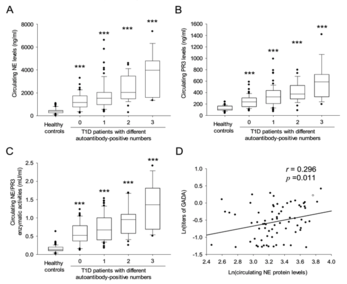
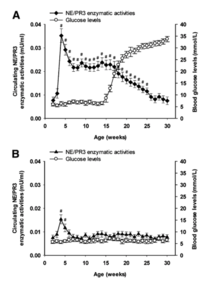
This invention provides antibodies and methods for early diagnosis of Type 1 diabetes by measuring the enzymatic activities and/or protein concentrations of serum neutrophil elastase (NE) and proteinase 3 (PR3) in a subject and comparing the measured levels of these proteases to reference levels. The increased enzymatic activities and concentrations of NE and PR3 are indicative of an increased risk or propensity of developing autoimmune diabetes. More importantly, an obvious elevation of NE and PR3 is detected even in those autoantibody-negative patients.
Benefits
- Eligible for simple, convenient detection.
- More sensitive than autoantibodies as predictive biomarker for TID development.
- Elisa kits and chromogen-based assay for NE and PR3 are available
Potential Product and Services
- Elisa kit for diagnosis of TID
- A membrane-based mini-chip for simultaneous detection of several major auto-antigens for TID for diagnosis of TID
Development Status
Patents
- US Patent No. 9,625,460;
- EP Application No. 15798693.6 filed on 6 Dec 2016;
- CN Application No. 201580028896.7 filed on 30 Nov 2016
IP Status
- Patented
- Patent application submitted