The First Class of Inhibitors for YEATS Domain as Potential Therapeutic Agents for Acute Leukemia
- 領域
- Therapeutic Chemicals
- Reference No.
- IP00706
Key Problem and Market Opportunity
- Mixed lineage leukemia (MLL) is characterized by the presence of MLL fusion proteins that are the result of chromosomal translocations affecting the MLL gene at 11q23. MLL rearrangement may be seen in about 80% of infants presenting with acute leukemia in the first 6 months of life and may be found in 3% to 4% of adults with acute leukemia.
- MLL is a is a very aggressive blood cancer with poor prognosis. Despite the availability of advanced treatment methods on acute leukemia, the cure rates of MLL have been low over the past years. Therefore, targeted therapy specific for MLL shall be developed to cure this disease.
- The leukemia therapeutics market was valued at $6.3 billion in 2010 and is expected to reach $11.3 billion by 2020 at a CAGR of 3.84% between 2015 and 2020.
Key Advantages of the Technology
-
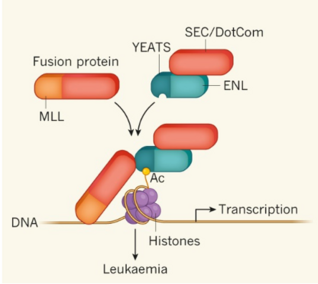
Two recent studies (Wan L., et al., Nature, 2017, doi: 10.1038/nature21687; Erb M. A., et al., Nature, 2017, doi: 10.1038/nature21688) have revealed the ENL YEATS domain as a novel therapeutic target for the treatment of MLL rearranged acute leukemia. The current invention provides the first group of small-molecule inhibitors targeting the YEATS domain which serve as good leads to develop therapeutic agents for the treatment of acute leukemia.
Benefits
-
The first class of inhibitors for the ENL YEATS domain as a novel therapeutic target
-
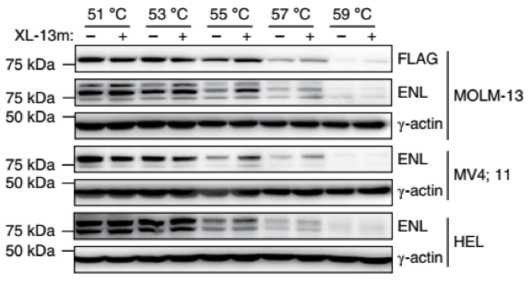
Selective engagement with endogenous ENL (tested pull-down assay and cellular thermal shift assays using MLL leukemia cell lines)
-
Disruption of ENL–chromatin interaction and inhibition of downstream oncogene transcription (tested by ChIP-qPCR assay using MLL leukemia cell lines)
Potential Product and Services
- Anti-leukemia drug targeting mixed lineage leukemia
Development Status and IP Strength
Stage of Development
- Lead compounds selected and their biological activities have been verified in vitro
Patents
- US Provisional Application No. 62/590,690 filed on 27 Nov, 2017.
IP Status
- Provisional patent